Studies on the mode of action
Various effects of electrostimulation on the retina have been described in the scientific literature1-14: Electrical impulses trigger biochemical signalling pathways that control physiological processes – in this case in the cells of the retina. Neuroprotective effects occur and this protective effect can halt or at least slow down the progression of retinitis pigmentosa (RP) and other degenerative retinal diseases.
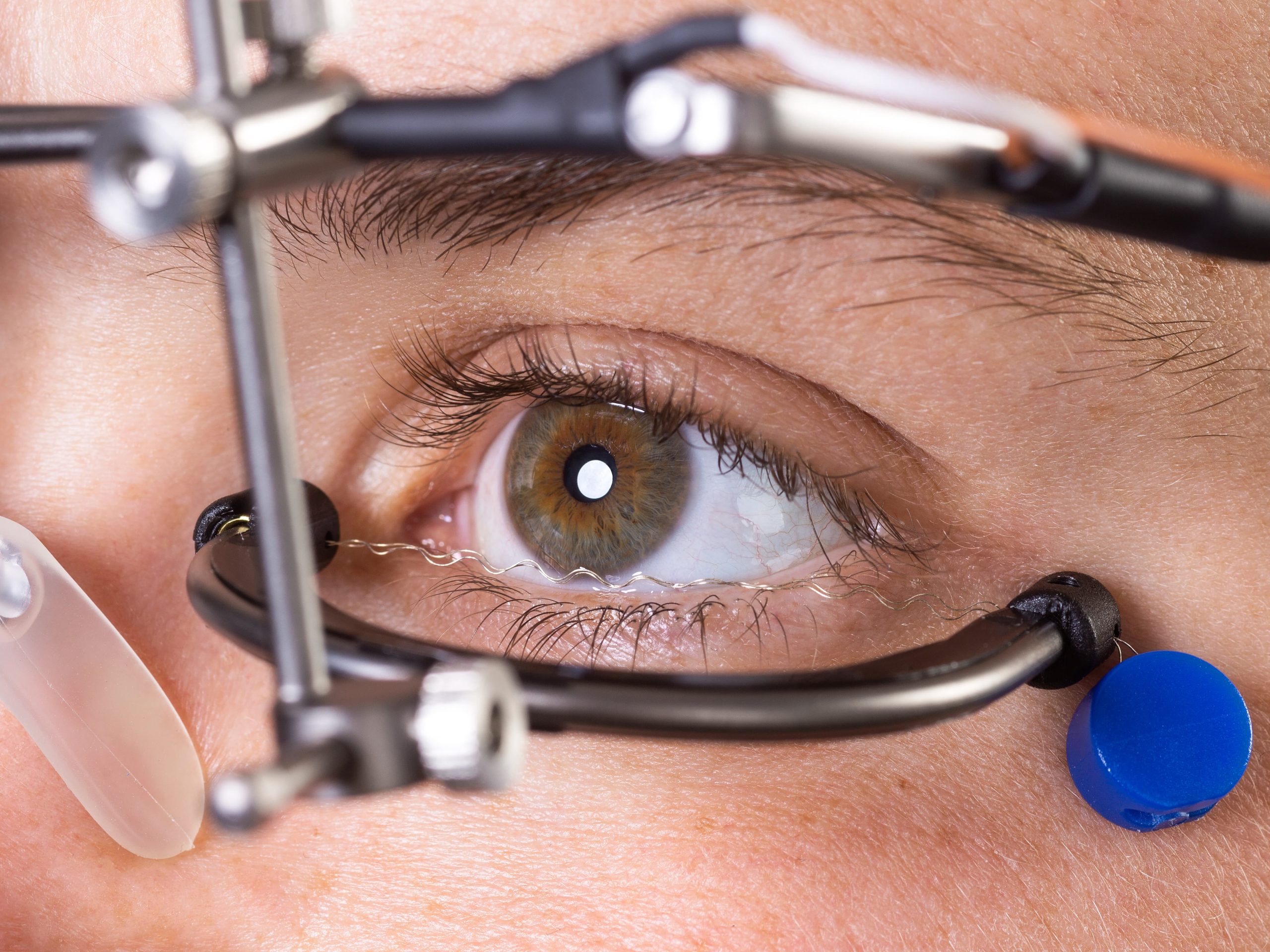
Studies confirm the safety and efficacy of OkuStim therapy
In 2011, a first clinical trial (EST1) with RP patients showed that retinal electrostimulation is effective and safe15. A follow-up study (EST2) and an observational study (TESOLA) confirmed the results in 2014/201616,17.
A recent exploratory analysis of the data collected in 2014 now provides further evidence of the effectiveness of OkuStim therapy. The data show that after one year of TES treatment, visual field deterioration in patients with retinitis pigmentosa (RP) was slowed down depending on the level of stimulation21.
Safe to use
All study patients taken together, the OkuStim treatment has already had over 130 years of use without any safety-related incidents. The safety was confirmed, among other things, by the above-mentioned observational study17.
In addition, the Clinical Working Group (AKF) of the Scientific Medical Advisory Board of PRO RETINA Deutschland e. V. regards the use of the OkuStim system as safe and has no objections against the controlled use in patients with retinitis pigmentosa and other generalised hereditary retinal dystrophies such as cone-and-rod dystrophies, choroideremia or Usher syndrome.
Effective use
Several studies showed the cell-preserving effects of electrostimulation, which can slow down disease progression (you can find a summary article here18). In particular, the larger long-term study EST216 suggests a reduction in visual field loss with electrostimulation. The response of the retina to short flashes of light, studied with electroretinography, or ERG, also showed positive effects.
With the publication of an extended analysis of the EST2 data, the dose-dependent slowing of visual field loss by TES has now been described for the first time21.
In addition, a retrospective study of about 100 patients, not conducted by Okuvision, shows a statistically significant positive effect of electrostimulation on the visual field19. The interesting thing here is that this positive influence was no longer detectable after 6 months without treatment. This suggests that the treatment with the OkuStim system should be applied as permanently as possible and without long interruptions.
State of Art
Since 2021 electrostimulation is mentioned as a treatment option in the current guideline for hereditary diseases of the retina, choroid and optic pathway of the German Opthalmological Society (DOG), the Retinological Society (RG) and the Professional Association of German Ophthalmologists (BVA).
Current studies
Observational study
In summer 2021, began a second large-scale observational study with patients using the OkuStim system. All OkuStim users who have been stimulating for at least one year or have stimulated for at least one year could participate.
The data collection on the objective and subjective long-term benefits of OkuStim therapy has now been completed and is being analysed. Results are expected next year.
The data shall complement the previous results on effectiveness. It should help the scientists and doctors as well as us as developers and manufacturers of the OkuStim system to further improve electrostimulation for you, the patients.
Ongoing clinical study
In order for retinal electrostimulation to be included in the benefits catalogue of the statutory health insurance funds in Germany, more patient data on its efficacy is being collected over a longer period of time. The benefit assessment is the goal of the trial study that was started under the direction of the University Eye Hospital in Tübingen20. The entire treatment costs will be covered by the German statutory health insurance funds for the duration of the trial.
Recruitment for this study has ended. First results can be expected in 2026.
Have we sparked your interest in more details about the studies and the effect of treatment with the OkuStim system? Then we will be happy to send you more information:
In addition you can find:
– Links to the individual studies: see below LINKS in References
– Information on the current TES-RP study on retinanet.de
References:
- Morimoto T, Miyoshi T, Matsuda S, Tano Y, Fujikado T, Fukuda Y. Transcorneal electrical stimulation rescues axotomized retinal ganglion cells by activating endogenous retinal IGF-1 system. Invest Ophthalmol Vis Sci. 2005 Jun;46(6):2147-55.
- Geremia NM, Gordon T, Brushart TM, Al-Majed AA, Verge VM. Electrical stimulation promotes sensory neuron regeneration and growth-associated gene expression. Exp Neurol. 2007 Jun;205(2):347-59.
- Sato T, Fujikado T, Lee TS, Tano Y. Direct effect of electrical stimulation on induction of brain-derived neurotrophic factor from cultured retinal Müller cells. Invest Ophthalmol Vis Sci. 2008 Oct;49(10):4641-6.
- Schmid H, Herrmann T, Kohler K, Stett A. Neuroprotective effect of transretinal electrical stimulation on neurons in the inner nuclear layer of the degenerated retina. Brain Res Bull. 2009 Apr 6;79(1):15-25.
- Ciavatta VT, Kim M, Wong P, Nickerson JM, Shuler RK Jr, McLean GY, Pardue MT. Retinal expression of Fgf2 in RCS rats with subretinal microphotodiode array. Invest Ophthalmol Vis Sci. 2009 Oct;50(10):4523-30.
- Ni YQ, Gan DK, Xu HD, Xu GZ, Da CD. Neuroprotective effect of transcorneal electrical stimulation on light-induced photoreceptor degeneration. Exp Neurol. 2009 Oct;219(2):439-52.
- Morimoto T, Kanda H, Kondo M, Terasaki H, Nishida K, Fujikado T. Transcorneal electrical stimulation promotes survival of photoreceptors and improves retinal function in rhodopsin P347L transgenic rabbits. Invest Ophthalmol Vis Sci. 2012 Jun 28;53(7):4254-61.
- Zhou WT, Ni YQ, Jin ZB, Zhang M, Wu JH, Zhu Y, Xu GZ, Gan DK. Electrical stimulation ameliorates light-induced photoreceptor degeneration in vitro via suppressing the proinflammatory effect of microglia and enhancing the neurotrophic potential of Müller cells. Exp Neurol. 2012 Dec;238(2):192-208.
- Osako T, Chuman H, Maekubo T, Ishiai M, Kawano N, Nao-I N. Effects of steroid administration and transcorneal electrical stimulation on the anatomic and electrophysiologic deterioration of nonarteritic ischemic optic neuropathy in a rodent model. Jpn J Ophthalmol. 2013 Jul;57(4):410-5.
- Ozeki N, Shinoda K, Ohde H, Ishida S, Tsubota K. Improvement of visual acuity after transcorneal electrical stimulation in case of Best vitelliform macular dystrophy. Graefes Arch Clin Exp Ophthalmol. 2013 Jul;251(7):1867-70.
- Fu L, Lo AC, Lai JS, Shih KC. The role of electrical stimulation therapy in ophthalmic diseases. Graefes Arch Clin Exp Ophthalmol. 2015 Feb;253(2):171-6.
- Kanamoto T, Souchelnytskyi N, Kurimoto T, Ikeda Y, Sakaue H, Munemasa Y, Kiuchi Y. Proteomic study of retinal proteins associated with transcorneal electric stimulation in rats. J Ophthalmol. 2015;2015:492050.
- Hanif AM, Kim MK, Thomas JG, Ciavatta VT, Chrenek M, Hetling JR, Pardue MT. Whole-eye electrical stimulation therapy preserves visual function and structure in P23H-1 rats. Exp Eye Res. 2016 Aug;149:75-83.
- Della Volpe-Waizel M, Zuche HC, Müller U, Rickmann A, Scholl HPN, Todorova MG. Metabolic monitoring of transcorneal electrical stimulation in retinitis pigmentosa. Graefes Arch Clin Exp Ophthalmol. 2020 Jan;258(1):79-87. LINK
- Schatz A, Röck T, Naycheva L, Willmann G, Wilhelm B, Peters T, Bartz-Schmidt KU, Zrenner E, Messias A, Gekeler F. Transcorneal electrical stimulation for patients with retinitis pigmentosa: a prospective, randomized, sham-controlled exploratory study. Invest Ophthalmol Vis Sci. 2011 Jun 23;52(7):4485-96. LINK
- Schatz A, Pach J, Gosheva M, Naycheva L, Willmann G, Wilhelm B, Peters T, Bartz-Schmidt KU, Zrenner E, Messias A, Gekeler F. Transcorneal Electrical Stimulation for Patients With Retinitis Pigmentosa: A Prospective, Randomized, Sham-Controlled Follow-up Study Over 1 Year. Invest Ophthalmol Vis Sci. 2017 Jan 1;58(1):257-269. LINK
- Jolly JK, Wagner SK, Martus P, MacLaren RE, Wilhelm B, Webster AR, Downes SM, Charbel Issa P, Kellner U, Jägle H, Rüther K, Bertelsen M, Bragadóttir R, Prener Holtan J, van den Born LI, Sodi A, Virgili G, Gosheva M, Pach J, Zündorf I, Zrenner E, Gekeler F. Transcorneal Electrical Stimulation for the Treatment of Retinitis Pigmentosa: A Multicenter Safety Study of the OkuStim® System (TESOLA-Study). Ophthalmic Res. 2020;63(3):234-243. LINK
- Liu J, Tong K, Lin Y, Lee VWH, So KF, Shih KC, Lai JSM, Chiu K. Effectiveness of Microcurrent Stimulation in Preserving Retinal Function of Blind Leading Retinal Degeneration and Optic Neuropathy: A Systematic Review. Neuromodulation. 2021 May 13. doi: 10.1111/ner.13414. Epub ahead of print. PMID: 33984873.
- Sinim Kahraman N, Oner A. Effect of Transcorneal Electrical Stimulation on Patients with Retinitis Pigmentosa. J Ocul Pharmacol Ther. 2020 Oct;36(8):609-617. LINK
- Kahle N, Peters T, Braun A, Franklin J, Michalik C, Gekeler F, Wilhelm B; retina.net e. V.; TES-RP-Studiengruppe. Transkorneale Elektrostimulation bei Retinitis pigmentosa : Prüfplan einer multizentrischen, prospektiven, randomisierten, kontrollierten und doppelblinden Studie im Auftrag des Gemeinsamen Bundesausschusses (G-BA-Erprobungsrichtlinie) [Transcorneal electrostimulation in retinitis pigmentosa : Protocol of a multicentric prospective, randomized, controlled and double-masked trial on behalf of the Joint Federal Committee (G-BA pilot regulation)]. 2021 May;118(5):512-516. LINK
- Stett A, Schatz A, Gekeler F, Franklin J. Transcorneal Electrical Stimulation Dose-Dependently Slows the Visual Field Loss in Retinitis Pigmentosa. Transl Vis Sci Technol. 2023;12(2):29. doi:10.1167/tvst.12.2.29 LINK